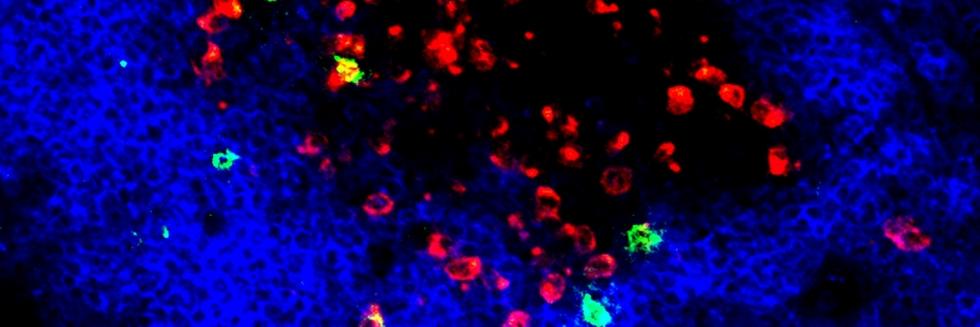
Regulatory T cells in skin mediate immune privilege of the hair follicle stem cell niche
Cohen JN, Gouirand V, Macon CE, Lowe MM, Boothby IC, Moreau JM, Gratz IK, Stoecklinger A, Weaver CT, Sharpe AH, et al. Regulatory T cells in skin mediate immune privilege of the hair follicle stem cell niche. Sci Immunol. 2024;9 (91) :eadh0152.Abstract
Immune tolerance is maintained in lymphoid organs (LOs). Despite the presence of complex immune cell networks in non-LOs, it is unknown whether self-tolerance is maintained in these tissues. We developed a technique to restrict genetic recombination to regulatory T cells (Tregs) only in skin. Selective depletion of skin Tregs resulted in T cell-mediated inflammation of hair follicles (HFs). Suppression did not rely on CTLA-4, but instead on high-affinity interleukin-2 (IL-2) receptor expression by skin Tregs, functioning exclusively in a cell-extrinsic manner. In a novel model of HF stem cell (HFSC)-driven autoimmunity, we reveal that skin Tregs immunologically protect the HFSC niche. Finally, we used spatial transcriptomics to identify aberrant IL-2 signaling at stromal-HF interfaces in a rare form of human alopecia characterized by HFSC destruction and alopecia areata. Collectively, these results reveal the fundamental biology of Tregs in skin uncoupled from the systemic pool and elucidate a mechanism of self-tolerance.
Read more