Regulatory T cells in dominant immunologic tolerance
Georgiev P, Benamar M, Han SJ, Haigis MC, Sharpe AH, Chatila TA. Regulatory T cells in dominant immunologic tolerance. J Allergy Clin Immunol. 2024;153 (1) :28-41.Abstract
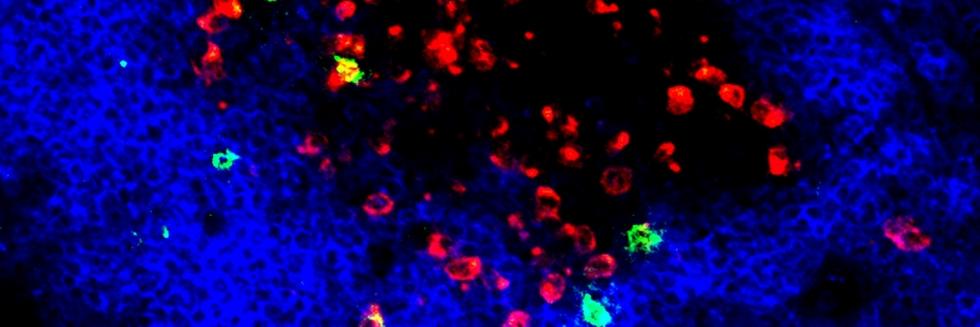
Regulatory T cells expressing the transcription factor forkhead box protein 3 mediate peripheral immune tolerance both to self-antigens and to the commensal flora. Their defective function due to inborn errors of immunity or acquired insults is associated with a broad range of autoimmune and immune dysregulatory diseases. Although their function in suppressing autoimmunity and enforcing commensalism is established, a broader role for regulatory T cells in tissue repair and metabolic regulation has emerged, enabled by unique programs of tissue adaptability and specialization. In this review, we focus on the myriad roles played by regulatory T cells in immunologic tolerance and host homeostasis and the potential to harness these cells in novel therapeutic approaches to human diseases.
Read more